WPs - Deliverables & Milestones
START: 1 January 2023
END: 31 December 2025
DURATION: 36 months
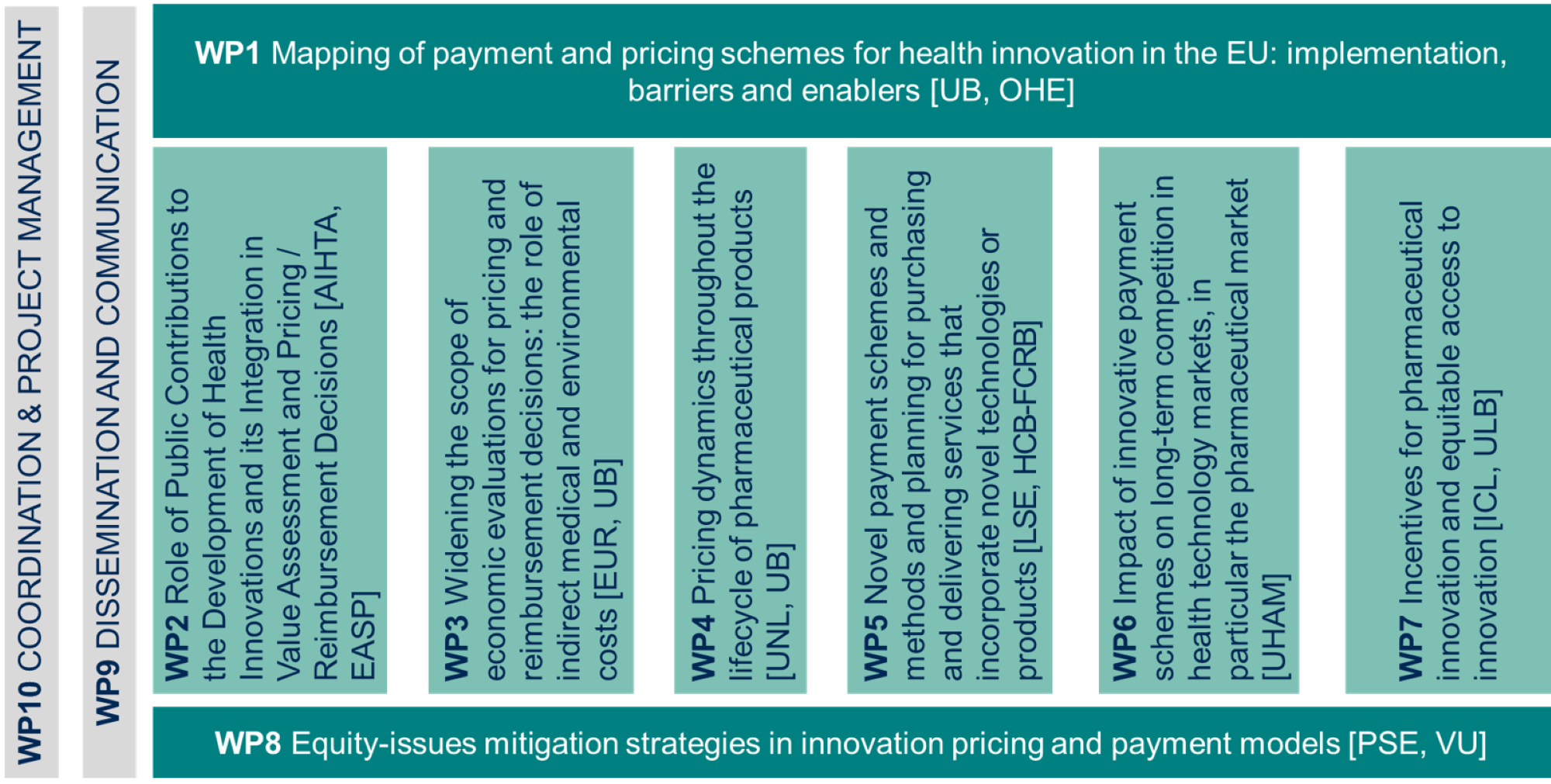
HI-PRIX work plan is organized into 10 work packages. WPs from 1 to 8 are scientific WPs
WP1
Mapping of payment and pricing schemes for health innovation in the EU: implementation, barriers and enablers
Deliverables
- D 1.1 Stakeholders judgement on barriers and enablers of novel payment/pricing schemes
- D 1.2 Policy recommendations about successful and flexible implementation of the different schemes to promote access to high-quality affordable innovative health technologies
Milestones
- M 1.1 “Pay for innovation Observatory” – an online catalogue of pricing and payment schemes for health innovation
- M 1.2 Within-country performance of novel payment/pricing schemes: costs and benefits of implementation
WP2
Role of Public Contributions to the Development of Health Innovations and its Integration in Value Assessment and Pricing / Reimbursement Decisions
Deliverables
- D 2.1 Guidance (Handbook) on estimations (ranges) of cost elements along the value chain for demanding detailed information in price negotiations
- D 2.2 Set of recommendations for using the public investment in the negotiation/HTA process and pilot example how price can be different if public investment is considered
Milestones
- M 2.1 Conceptual frameworks with recommendations on public interventions aimed at fostering efficient health innovation at a fair cost for society
WP3
Widening the scope of economic evaluations for pricing and reimbursement decisions: the role of indirect medical cost and environmental impact
Deliverables
- D 3.1 Equity implications of including indirect medical costs and environmental impacts in economic evaluations informing pricing and reimbursement
- D 3.2 Policy guide on the role of cost-effectiveness and budget impact analyses (including indirect medical costs and environmental impacts) in pricing and reimbursement decisions in different European decision contexts
Milestones
- M 3.1 Literature review of role of cost-effectiveness and budget impact in pricing and reimbursement decisions and the role of indirect medical costs therein
- M 3.2 Literature review on the approaches and consequences of including environmental impacts in pricing and reimbursement based on economic evaluations
- M 3.3 European tool for estimating and including indirect medical costs and environmental impacts in economic evaluation, freely available, including manual
M 3.4 Impact of including indirect medical costs and environmental impacts in economic evaluations and budget impact analysis and potential implications for pricing and reimbursement using two case studies
Download PDF here
WP4
Pricing dynamics throughout the lifecycle of pharmaceutical products
Deliverables
- D 4.1 A novel dynamic pricing model that links to clinical benefit: impact and acceptability for stakeholders
- D 4.2 Recommendations on pricing principles for multi-indication products and conditions for their successful implementation
Milestones
- M 4.1 Do prices evolve in response of new clinical evidence generated post-launch? Real-world examples from oncology drugs authorized via accelerated review
- M 4.2 Price discrimination with multi-indication products: underpinning economic theory and empirical evidence
Download PDF here:
Milestone 9 WP4
WP5
Novel payment schemes and methods and planning for purchasing and delivering services that incorporate novel technologies or products
Deliverables
- D 5.1 Impact of applying the new payment schemes to (a) a primary care and (b) an integrated care setting
- D 5.2 Assessment of transferability and generalizability of results and development of a toolkit for decision-makers
Milestones
- M 5.1 Key components in payment schemes for health care innovations included in health system provision
Download PDF here:
Milestone 10 WP5
WP6
Impact of innovative payment schemes on longterm competition in health technology markets, in particular the pharmaceutical market
Deliverables
- D 6.1 Report on contextualization of simulation results and synthesis of findings with regard to the current use and the regulatory measures, levers and barriers to the widespread practical implementation of the respective payment schemes and their impact on long-term competition in health technology markets
Milestones
- M 6.1 Results of simulation on thew impact of various payment chemes on cost differences for both, manufactures and society, specifically focusing on different sources of uncertainty
Download PDF here:
Milestone 11 WP6
WP7
Incentives for pharmaceutical innovation and equitable access to innovation
Deliverables
- D 7.1 Effectiveness and equity implications of selected pharmaceutical innovation policies and incentive mechanisms
- D 7.2 Strength of incentive mechanisms and policy recommendations to incentivize pharmaceutical innovation in key areas of need
Milestones
- M 7.1 Database with detailed mapping of policies and incentive mechanism to foster pharmaceutical innovation and access to innovation
WP8
Equity-Issues Mitigation Strategies in Innovation Pricing and Payment Models
- D 8.1 Outline of the equity-issues mitigation strategies identified, and related potential obstacles
- D 8.2 Report on the overall performance of a selection of equity-issues mitigation strategies and recommendations
WP9
Dissemination and communication
Deliverables
- D 9.1 Dissemination and Communication Plan
- D 9.2 Data Management Plan
- D 9.3 Updated DPM
- D 9.4 Launch of Project Website
Milestones
- M 9.1 HI-PRIX Final conference
WP10
Coordination and project management
Deliverables
- D 10.2 Project management book
Milestones
- M 10.1 HI - PRIX Yearly Consortium meetings